Our manifesto
Our manifesto
Accelerating drug development andempowering precision neurologyto improve brain care
Neurological conditions are the 1st cause of disability worldwide(1), affecting 1 on 3 people. This situation is increasing fast with ageing of population. But neurological diseases remain highly underserved.
Why? Drug development failure mostly comes from the lack of relevant and specific biomarkers to access brain microstructure.
BrainTale provides sensitive, reliable, clinically validated and easily accessible biomarkers to accelerate Central Nervous System (CNS) drugs development and optimize patient care.
By exploring better, we can treat faster, together
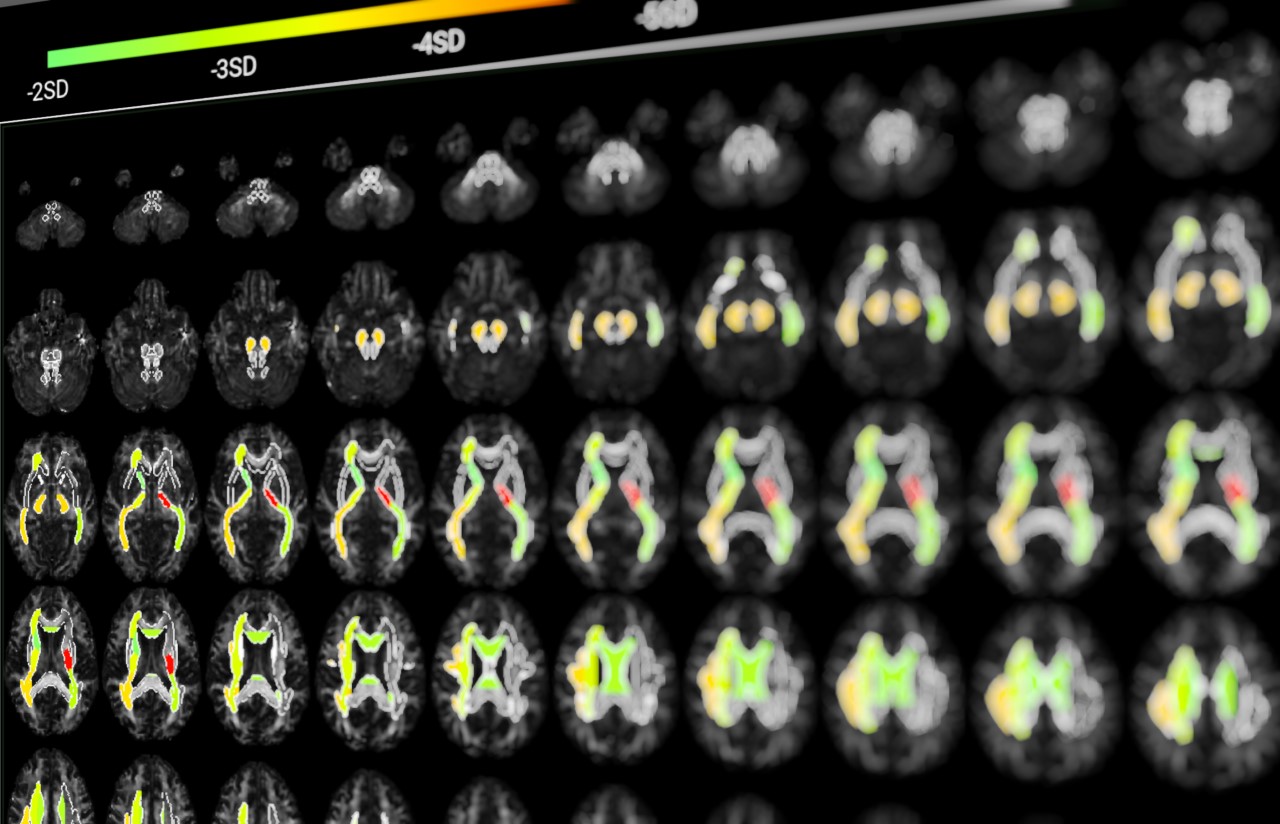
White matter is what matters
White matter understanding is underestimated despite representing 60% of the human brain. Its impairment is either a cause or a consequence of many, if not all, neurological conditions. Its exploration can lead to major discoveries in neurology, notably by improving disease diagnosis, accelerating the development of new disease-modifying therapies and bringing them to a successful conclusion/bringing them to fruition.
OUR GOAL: provide a new relevant and universal white matter measure such as degrees in thermometer.
We help to:
- – Identify patients at risk and provide early diagnosis information
- – Evaluate treatment efficacy
- – Monitor and follow-up disease evolution
- – Support decision-making in real-life settings
Therapeutic Areas
- – Demyelinating diseases such as adrenoleukodystrophies or multiple sclerosis
- – Neurodegenerative diseases such as Parkinson’s disease
- – Aging diseases such as Alzheimer’s disease
- – Traumatic conditions, such trauma brain injury or cardiac arrest.
Our approach is
- – Already actionable in the daily clinic
- – Non-invasive and painless
- – Clinically validated
Our platform of biomarkers
- – Is proprietary
- – Is used by physicians, hospitals and drug developers
- – Since 2022 under the MDR
- – Is CE-marked
Source :
BRAINTALE, whose head office is 11 rue de l’Académie 67000 – STRASBOURG, is registered with the Trade and Companies Register under number 840 995 138 RCS STRASBOURG
Last update : 09/07/2024

