Head traumas and the majority of neurological and psychiatric diseases, such as Alzheimer’s, Parkinson’s, Multiple Sclerosis, Adrenoleukodystrophy (ALD) or Amyotrophic Lateral Sclerosis are directly or indirectly linked to alterations of the white matter1.
Thus far, we have not identified any neurological condition that would not benefit from having an understanding of white matter integrity.
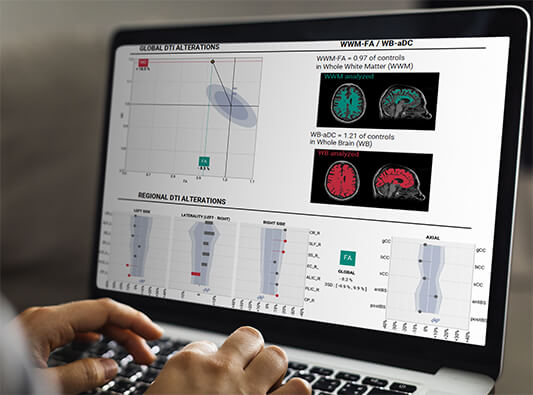
BrainTale-care platform provides reliable, reproducible and quantitative measurement of the brain
Our approach is organ-specific2.
Our products suite are based on brainTale-care platform
brainTale-care is a clinically evaluated software medical device available in SaaS (Software as a Service) mode for any physician with an access to a diffusion MRI:
-
We identify white matter alterations
-
We provide precision-medicine support to diagnostic and monitoring as well as score supporting prognosis1
-
Our ambition is to provide physicians, drug developers and hospitals with the power to provide better brain care and treatments for their patients
The BrainTale-care platform is made of two modules. Our brainQuant module is including MyelinDx. More modules are currently being developed by our team.
BrainQuant is being used both for white matter assessment daily as well as in clinical trials for improvement of drug developments
Use cases include potential diagnostic and monitoring of diseases1,4
MyelinDx
MyelinDx3 is one of the output data provided by brainQuant to reflect myelin lesions and enabling the lesions follow-up for efficient drug development1.
Provides a prediction score of coma recovery for comatose after cardiac arrest and trauma brain injuries5
Provides clinicians with reliable and unprecedented information to support intensive care decision-making
Our clients & partners
BrainTale-care platform is already used by
Legal disclaimer:
– BRAINTALE, whose head office is 11 rue de l’Académie 67000 – STRASBOURG, is registered in the Trade and Companies Register under number 840 995 138 RCS STRASBOURG
– Manufacturer address: 140 rue du Chevaleret, 75013 Paris
– BrainTale-care is a Class IIa medical device
– Notified body : BSI Netherlands 2797
– This information deals with brainTale-care platform and modules (brainQuant and brainScore-coma). We invite you to read carefully the instructions for use and the label.
– BrainTale-care is not reimbursed by social security.
– BrainTale is ISO 13485 certified company
![]()
Clinical warnings:
– The use of brainQuant can by no means replace a diagnosis by a licensed and competent physician, nor constitute medical advice or a diagnosis, which can only be obtained from a physician. The physician bears the sole responsibility for the diagnosis. The calculated parameters are subject to variability and cannot be used without medical advice from a licensed and competent physician.
– brainScore-coma is informing clinical management as part of a multimodal evaluation process, including several sources or technologies (clinical information, biological or electrophysiological parameters, imaging data including MRI/CT/PET) and is not triggering an immediate or near-term action by itself. It is not used to guide next diagnostics or next treatment interventions. The physician and the multidisciplinary team bear the sole responsibility for the patient management based on various information including prognostics tools.
– The use of brainScore-coma can by no means replace a diagnosis by a licensed and competent physician nor constitute medical advice or diagnosis which can only be obtained from a physician. The physician bears the sole responsibility for the diagnosis. The calculated parameters cannot be used without the medical advice of a licensed and competent physician.
– brainScore can lead to no outcome parameters due to the poor quality of MR images.
References:
1 Clinical evaluation braintale-care and technical file braintale-care platform and modules medical device
2brainQuant: A coefficient of variation in test-retest repeatability of the diffusion outcome parameters inferior to 10% and a coefficient of variation in test-retest in test-retest reproducibility of the diffusion parameters inferior to 15%.
brainScore-coma: Low variability: Coefficient of variation in test-retest repeatability of the prognosis outcome parameters inferior to 10%. Coefficient of variation in reproducibility across MRI scanners the prognosis outcome parameters inferior to 15%. Good level of certainty: Sensitivity and specificity of brainScore-coma. For cardiac arrest patient (WWMFA, prediction of 6-months Best CPC from 3 to 5 with 95% Confidence interval):
-
- Specificity = 100 % (89%-100%)
- Sensitivity = 89% (82% – 94%)
3 MyelinDx is the trade name of RD index19 according to our technical file.
4 brainQuant is intended to be used for adult subjects (> 18-year-old) as the brain atlases used for processing the MRI have been designed based on adult brains.
5 Comatose patients admitted in intensive care unit after cardiac arrest or head trauma.
– Age ≥18 (adult population, regardless of gender) – For cardiac arrest patient: between 18 and 80 years old (limits included).
– Persisting unconsciousness at day 7 after cardiac arrest or severe head trauma.
Last update : 20/10/2023





