Interview with Marc Martinell, CEO of Minoryx Therapeutics
We are proud to share a short interview with Marc Martinell, co-founder and CEO of Minoryx Therapeutics. This article discusses the fruitful partnership with Braintale. Over the past three years, this collaboration has used Braintale’s cutting-edge DTI- MRI technology to advance clinical research and improve diagnostics for rare brain diseases such as adrenoleukodystrophy. Marc shares his experience about how Braintale helps them develop therapies, explore physiopathology, the obstacles they face, and the potential for exciting new outcomes.
Learn more about how these two pioneering companies are working together to push the limits of medical research and patient care by reading this interview!

Can you tell us how you became involved with Braintale and the nature of your collaboration?
Marc Martinell: Braintale has been around for a while now. Our relationship began initially through collaboration on a clinical study where Braintale provided analytical support in a collaborative manner. A principal investigator in France was enthusiastic about their technology, which led to this initial connection. Over time, this collaboration evolved into a global partnership that encompasses both collaborative aspects and provisions that Braintale delivers for our past and ongoing studies.
For someone unfamiliar with Braintale, how would you describe their work and its significance?
Marc Martinell: Braintale is a company specializing in advanced nuclear magnetic resonance imaging techniques used in clinical research and diagnostics. Their technology (DTI) allows the identification and monitoring of patients efficiently within clinical trials, also providing information on treatment effect. This objective assessment is crucial for the successful recruitment and treatment of patients in our clinical development. However, in real-world settings, widespread adoption might be challenging due to the need for calibrated equipment: that’s a challenge Braintale is facing to extend as broadly as possible the potential of their technology.
What do you see as the primary value that Braintale brings to your work at Minoryx, and how do you envision this partnership evolving over the next five years?
Marc Martinell: The main benefit Braintale offers lies in their capability to convert a variety of information into understandable outcomes, which supports our thorough understanding of how treatments affect the brain. This is especially important for rare diseases, such as the ones we focus on, where precise and meaningful diagnostic tools are essential – both from a patient by patient and from a population perspective. Looking ahead, I believe Braintale should broaden the scope of their tech’s use to other brain conditions and clinical development. Their relevance and impact could be enhanced by doing so in both clinical research and broader medical applications.
How would you describe your interactions with the Braintale team? Which values do they bring to the table?
Marc Martinell: The Braintale team is very approachable, humble, and transparent. We regularly interact with key members like Julie, Vincent, and Marine, who manage our projects. Their openness and clarity make collaboration smooth and productive. They are very committed to their work and ensure that their technology meets the highest standards of quality and excellence.
In your opinion, what are the key success factors that would convince other pharma players to partner with Braintale?
Marc Martinell: Braintale’s strength lies in their ability to transform complex MRI data and distill it into meaningful, actionable insights. This capability is particularly valuable for pharmaceutical companies looking to monitor the efficacy of their treatments on brain conditions. Additionally, Braintale’s collaborative approach and their support in understanding and interpreting data truly supports the partnership spirit.
Hepatic encephalopathy: a new study proves the relevance of white matter biomarkers
Strasbourg, June 7th, 2024
- “Cerebral diffusion tensor imaging in patients with liver diseases admitted for evaluation of neurological symptoms” is presented today at the EASL congress.
- Those first data pave the way for the interest of white matter monitoring with BrainTale’s biomarker platform in hepatic encephalopathy patients’ management, a common complication of liver diseases.
BrainTale, a medtech deciphering white matter to enable better brain care, spin-off of the Paris Region Greater Hospitals, presented the results of the “Cerebral diffusion tensor imaging in patients with liver diseases admitted for evaluation of neurological symptoms” study led by Professor Nicolas Weiss, (Sorbonne University, Paris, France) during the European Association for the Study of Liver (EASL) congress in Milan (Italy) from June 05 to 08, 2024. The presented scientific communication paves the way for the interest of white matter monitoring with BrainTale’s biomarker platform in hepatic encephalopathy patients’ management, a complication of liver disease.
Long underestimated in neuroscience, white matter, which represents 60% to 80% of the human brain, plays a key role in its proper functioning, development, and aging, whether normal or pathological. Accordingly, since its creation in 2018, BrainTale has been developing non-invasive, accessible, actionable in multicentric frameworks, and clinically validated measurement and predictive tools for patients suffering from brain diseases. With a strong collaborative approach, the company’s ambition is to provide investigators with relevant measure of the brain to improve patient care and clinical development of new disease-modifying drugs.
Hepatic encephalopathy is a frequent complication of liver disease, characterized by deterioration in cerebral function. In 30-45% of the cases, the disease is symptomatic with clinical signs, yet recent animal data have suggested that several episodes of OHE (overt hepatic encephalopathy) may be associated with cerebral white matter lesions. With BrainTale solution, such lesions can now be identified, quantified, differentiated (axonal vs. myelin) and followed over time hence offering efficient non-invasive and objective diagnosis. It represents a possible breakthrough for patients’ management.
The scientific communication “Cerebral diffusion tensor imaging in patients with liver diseases admitted for evaluation of neurological symptoms” highlighted the study carried out on chronic liver disease patients with neurocognitive symptoms and was presented on June 7th during the session “Cirrhosis and its complications: Other clinical complications except ACLF and critical illness” at 8:30 am by Nicolas Weiss.
Of the 164 included patients, 118 (94 with cirrhosis and 24 with liver vascular disease) presented with a brain abnormality and underwent DTI sequence acquisition analyzed using BrainTale’s solution. It provided global fractional anisotropy (FA) and mean diffusivity (MD). Results showed that FA and MD are significantly impaired in liver disease patients with neurological symptoms. Cirrhotic patients had lower FA and higher MD values (p<0.05), and both markers correlated with neuropsychological tests. In the long term, a decrease in FA and an increase in MD were positively correlated with the number of OHE episodes.
These preliminary data highlight the relevance of white matter biomarkers available with BrainTale’s technology and demonstrate the company’s collaborative approach to improve patient care.
« Presented data enable the opening of a new perspective in OHE patient care with non invasive, multicentric and reliable approach to brain alterations monitoring” comments Professor Nicolas Weiss. “The objective assessment provided by white matter understanding with Braintale complements the neuropsychological testing, reducing heterogenicity and optimizing ultimately patient care. It could lead to a better understanding of hepatic encephalopathy” he adds.
About BrainTale
BrainTale is an innovative European medtech company measuring the brain through white matter quantification and standardization based on a commercially available software medical device solution. This regulatory-cleared AI software offers quality controlled, non-invasive, reliable and clinically validated reports after diffusion tensor MRI data processing. BrainTale enables drug developers, leading academic researchers and physicians to improve patient care by understanding neuroinflammatory and neurodegenerative conditions, assess brain lesions evolutions and take appropriate decisions in the clinic and during drug development. BrainTale empowers the scientific and medical community with objective measure to transform brain care.
Because brain diseases have become the medical issue of our time, we can no longer wait. At BrainTale, we strongly believe that by exploring better, we can treat faster, together.
For more information, please visit www.braintale.eu
Contacts
BrainTale – Lisa Marcheval, communication manager, lisa.marcheval@braintale.eu, +336 01 79 19 05
Press Relations - Anna Casal, casal.anna@gmail.com, +336 50 61 55 71
BrainTale accelerates its international expansion and unveils tier-one collaborations to improve brain care and enable precision neurology
Paris, May 6th, 2024
– BrainTale unveils partnerships with leading research centers in Europe and in the US to ultimately improve brain care through biomarkers development.
– By powering precision neurology, the company provides the brain research community with relevant quantitative imaging biomarkers to improve patient care and clinical development of new disease-modifying drugs.
BrainTale, a medtech deciphering white matter to enable better brain care, spin-off of the Paris Region Greater Hospitals, unveils today a selection of leading research centers collaborations. For its partners, BrainTale offers innovative regulatory-cleared software medical device combining relevant standardized white matter quantification and AI solutions. Its final goal is to assess brain damages evolutions with a high efficiency and accuracy in neuroinflammatory and neurodegenerative conditions to finally take appropriate decisions, both in the day-to-day clinic and during drug development.
Long underestimated in neuroscience, white matter, which represents 60% to 80% of the human brain, plays a key role in its proper functioning, development, and aging, whether normal or pathological. Accordingly, since its creation in 2018, BrainTale has been developing non-invasive, accessible, actionable and clinically validated measurement and predictive tools for patients suffering from brain diseases. With a strong collaborative approach, the company’s ambition is to provide investigators with relevant measure of the brain to improve patient care and clinical development of new disease-modifying drugs.
BrainTale discloses a selection of its partners research centers of excellence
With Professor Fanny Mochel, MD, PhD in charge of the reference center for Neurometabolic Diseases and Leukodystrophy at Hôpital de la Pitié-Salpêtrière, Assistance Publique – Hôpitaux de Paris (Paris, France), the collaboration focuses notably on leukodystrophies. Professor Mochel comments: “Implementing BrainTale’s biomarkers in our daily clinical practice and integrating this white matter quantification method in the clinical development brings significant value to our practice. Our expert center aims at providing the right treatment at the right time, and BrainTale’s markers offer invaluable insights and benefits toward achieving this”.
With Professors Ayham Alkhachroum, MD, MSc, Associate Professor of Neurology and Neurocritical Care and Sebastian Koch, MD, Professor of Clinical Neurology at the University of Miami Miller School of Medicine (Miami, Florida, USA), the collaboration will assess the benefits of BrainTale’s markers to evaluate patient after acute cerebral hemorrhage. Professor Alkhachroum comments: “There is an unmet clinical need to improve patient’s management in acute cerebral hemorrhage by selecting patients who can benefit from surgical evacuation. BrainTale’s technology can provide key information to advance decisions toward improving care in acute cerebral hemorrhage patients”.
Professor Rajiv Gupta is an Associate Professor at Harvard Medical School, Medical Director at MGB Enterprise Neuroradiology, Mass General Brigham, Vice Chair for Clinical Operations in the Department. of Radiology at the Massachusetts General Hospital (Boston, Massachusetts, USA). He is a member of BrainTale’s Scientific Advisory Board with focus on traumatic injuries and ageing diseases. ”Working with BrainTale empowers our research with standardized and calibrated DTI metrics. It paves the way for widespread diffusion of such biomarker both in research and in clinical practice”, comments Professor Gupta.
An innovative, highly powerful tool and decisive enabler for caregivers and drug developers
BrainTale aims at contributing to a better understanding of underlying mechanisms of several diseases and conditions, such as cognitive disorders including Alzheimer’s disease, demyelinating diseases such as leukodystrophies and multiple sclerosis, and acute conditions such as traumatic brain injuries or stroke.
The non-invasive white matter quantification provided by BrainTale is an innovative, highly powerful tool and decisive enabler for caregivers and drug developers, offering objective assessment to refine patient populations and monitor individual or patient population responsiveness to new therapies in development. By facilitating early detection, target identification, patient stratification, and treatment monitoring, biomarkers enhance the efficiency, effectiveness, and safety of drug development efforts in the central nervous system complex and challenging therapeutic environment. BrainTale’s biomarkers relevance in contemplated conditions has been heavily supported by fast growing scientific literature in the recent years. In practice, BrainTale’s software solution offers quality controlled, reliable, standardized and clinically validated reporting of diffusion MRI data processing that dramatically reduce multicenter variability from any MRI scanner type in standard clinical setting.
« Our collaborative approach is the foundation of BrainTale’ strategy to establish white matter measure as a reference to ultimately contribute to a better brain care. We are thrilled to unveil those first partnerships and intend to expand our network of partners in the coming months”, comments Vincent Perlbarg, BrainTale’s chief scientific officer and cofounder.
About BrainTale
BrainTale is an innovative European medtech company measuring the brain through white matter quantification and standardization based on a commercially available software medical device solution. This regulatory-cleared AI software offers quality controlled, non-invasive, reliable and clinically validated reports after diffusion tensor MRI data processing. BrainTale enables drug developers, leading academic researchers and physicians to improve patient care by understanding neuroinflammatory and neurodegenerative conditions, assess brain lesions evolutions and take appropriate decisions in the clinic and during drug development. BrainTale empowers the scientific and medical community with objective measure to transform brain care.
Because brain diseases have become the medical issue of our time, we can no longer wait. At BrainTale, we strongly believe that by exploring better, we can treat faster, together.
For more information, please visit www.braintale.eu
Contacts
BrainTale – Lisa Marcheval, communication manager, lisa.marcheval@braintale.eu
Press relations: Anna Casal +33650615571 casal.anna@gmail.com
Three questions for Braintale on... Multiple Sclerosis
The necessity of accurately and reliably measuring the brain’s white matter is crucial for monitoring patients with various pathologies, particularly multiple sclerosis (MS). Professor Damien Galanaud, a neuroradiologist at Assistance Publique Hopitaux de Paris – Hopital de la Pitié Salpêtrière, explains the importance of this measurement for assessing the diffuse damage to white matter, an aspect often overlooked by conventional monitoring methods.
When did you start introducing in your practice white matter measures as provided by Braintale?
Pr. Damien Galanaud: As a co-founder of Braintale, I have been using this solution since it first became commercially available in 2021. Initially, it was very useful for me in cases of comas, especially for assisting in prognosis determination. Today, I also use it for monitoring patients with adrenoleukodystrophy, a rare neuro metabolic disease. Its physiopathology is very similar to multiple sclerosis, and so the next step will be to use it to monitor this much more common pathology.
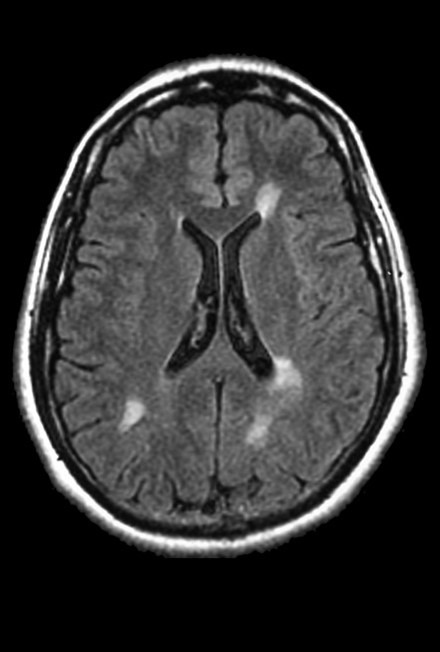
What is the main pain point in MS patient monitoring?
Pr. Damien Galanaud: Measuring white matter using diffusion tensor MRI, as proposed by Braintale, offers significant advantages in two respects for monitoring MS patients.
First, current measurements are based essentially on the progression of the number of plaques and take very little account of diffuse white matter damage only currently assessed by evaluating cerebral atrophy, a very late marker of the disease. The advantage of precise, standardized measurement of white matter is to ensure that patients are well controlled, both by halting the increase in the number of plaques and by curbing diffuse damage even before the onset of atrophy.
The second benefit of this measurement is in monitoring progressive forms of MS, which have very few plaques but are primarily characterized by diffuse, degenerative white matter damages. Currently, we are at a loss with these patients, whose condition worsens without us being able to measure any significant change with conventional sequences.
In the first case, white matter measurement is truly complementary to the current approach. In the second case, it is a truly innovative, groundbreaking information that could change significantly how patients are being taken care of.
Source : https://multiplesclerosisnewstoday.com/multiple-sclerosis-diagnosis/mri-magnetic-resonance-imaging/
How easy is it to assess white matter measure?
Pr. Damien Galanaud: White matter and its role in neuroinflammatory and neurodegenerative diseases are widely studied by researchers but its quantitative assessment remains challenging in clinical practice. Until now, standardized white matter quantification has not been integrated in the standard of care as no FDA/CE solution was available. The power of machine calibration is key to obtain reliable and reproducible measures from one scan to another, thus enabling multi-scan analysis and, therefore, take appropriate decision from a medical standpoint. This equation is solved here!
On a more practical level, this measure is obtained through a standard diffusion tensor MRI examination, which does not involve any supplementary exam as already included in the patient’s journey – either in clinical trials or in the patient care. It does not add some extra time for the doctor or the patient. It’s an easy sequence to add to the standard protocol, making it a powerful, reliable, and efficient tool.
Three questions for Braintale on… Alzheimer's disease
What is the nature of Alzheimer’s disease and its impact on the brain from a neurological perspective?

Arthur Bezie, Senior Brand Manager Braintale: Alzheimer’s disease is a neurodegenerative condition characterized by the progressive degeneration of neurons, particularly in the limbic cortex, hippocampus, and entorhinal cortex, leading to impacts on memory. Although the exact mechanisms remain poorly understood, two molecules play a central role: beta-amyloid peptide, which accumulates in toxic plaques between neurons, and Tau protein, responsible for neurofibrillary degeneration inside neurons. The disease gradually spreads to other brain regions, affecting various functions, including cognitive and behavioral ones.

Vincent Perlbarg, cofunder and Scientific Director Braintale: The white matter, crucial for neuronal connectivity, is closely linked to the disease as it is directly affected. Moreover, today, several scientific voices are questioning these complex connections because white matter also seems to play a significant role in disease progression, challenging therapeutic approaches focused solely on Tau and amyloid plaques. The white matter appears to be affected by the disease much earlier than previously thought, turning it into new potential therapeutic targets.
How long have you been working on Alzheimer’s disease at Braintale, and what motivated you to embark on this journey?
Vincent Perlbarg: Our team has been involved in Alzheimer’s fight for the past two years, corresponding to the increasing scientific awareness of white matter importance. This focus reflects both the evolution of scientific knowledge and Braintale’s preexisting solid expertise in white matter’s understanding .
Arthur Bezie: Our interest in Alzheimer’s also intensified through the analysis of scientific literature: we identified more than 800 scientific papers on the study of Alzheimer’s disease using diffusion MRI in the past 20 years! The trend has drastically accelerated in recent years with more than 60 papers published annually. This is huge! Since 2022, we gathered promising exploratory results from white matter measurements from available public databases. The urgency and relevance of our work given the crucial need for patients encourage us to continue even harder
What are Braintale’s contributions to the fight against Alzheimer’s disease, and how do you envision the future?
Vincent Perlbarg: So far, we have contributed to understanding Alzheimer’s disease mainly through dialogue with academic players and industrial actors, and analysis of existing data. This allowed us to confirm our biomarkers’ relevance in different contexts of use, such as early detection of the disease and monitoring of its progression. These are crucial for assessing treatment effectiveness, notably during drug development. We are now ready to partner with the industry to accelerate and secure clinical development and with physicians to optimize patient care. Our focus will be on identifying reliable biomarkers to enable efficient decision-making. Given the high failure rates in developing drugs for Alzheimer’s, the challenge is significant and we believe our solution will be instrumental in this journey.
Braintale's speeches in 2023
Braintale’s speeches in 2023
2023 was rich in discussions for Braintale: from collaborations to pathologies in neurology, from podcasts to conferences, Braintale was visible at every level! We invite you to (re)experience his various interventions through our LinkedIn publications:
March 2023

Vincent Perbarg, Braintale’s Scientific Director, presented the results of our biomarker platform at the ADPD congress on March 29, 2023:
https://www.linkedin.com/feed/update/urn:li:activity:7049772828933378048
June 2023

Braintale is the winner of the Fondation Force’s call for projects. Dorothée Uriet, Braintale’s former Regulatory Director, was in Strasbourg to speak at the awards ceremony on June 13, 2023:
https://www.linkedin.com/feed/update/urn:li:activity:7080090592696750080
Pr. Louis Puybasset, scientific advisor for Braintale, took part in the Réanimation congress on June 14 and 15, 2023 to present Braintale’s solution for coma patients:
https://www.linkedin.com/feed/update/urn:li:activity:7075025493288177664
On June 20, 2023, Braintale announced that its 4.5 million euros fundraising. Following this announcement, Madyness wanted to write an article featuring Julie Rachline, CEO of Braintale:
https://www.linkedin.com/feed/update/urn:li:activity:7077267606931431424

On June 22, 2023, Dipeeo organized a webinar on healthcare data. Our former regulatory director, Dorothée Uriet, shared her experience:
https://www.linkedin.com/feed/update/urn:li:activity:7075421350013624320
July 2023
Vincent Perlbarg, Braintale’s Scientific Director, presented our research results concerning Parkinson’s disease at the EAN and World Parkinson Congress on July 4 and 6, 2023:
https://www.linkedin.com/feed/update/urn:li:activity:7082996641330274304
Braintale has co-organized with its partner Effik the symposium on ALS disease at the ENCALS congress on July 12, 2023: https://www.linkedin.com/feed/update/urn:li:activity:7085158431304638464
August 2023
Prof. Rajiv Gupta, Braintale’s scientific advisor, presented a poster on Braintale biomarkers at the ENRS congress on August 25, 2023:
https://www.linkedin.com/feed/update/urn:li:activity:7102983389854461953
October 2023

Braintale is a committed player in the ALS disease. October 13, 2023, we invited the whole Braintale team, our shareholders and EFFIK to attend a private screening of Invincible Eté about Olivier Goy, while suffering from ALS:
On October 19, 2023, Effik, in collaboration with Braintale, wanted to revive the ENCALS symposium from July 2023:
https://www.linkedin.com/feed/update/urn:li:activity:7120378005230608384/

Braintale was invited on October 27, 2023 to participate in the roundtable about European Charter for Responsible Development of Neurotechnologies at Brain Innovation Days:
https://www.linkedin.com/feed/update/urn:li:activity:7123644965561208832/
November 2023
La Banque Postale organized the « Trophées de l’innovation 2023 », and Braintale won the « Coup de Cœur » prize. Julie Rachline, CEO of Braintale, represented the company during the awards ceremony on November 7, 2023:
https://www.linkedin.com/feed/update/urn:li:activity:7127977574470705152/
MACSF invited Julie Rachline, CEO of Braintale, to participate in an episode on the brain of its podcast “Ca ira mieux demain”:
https://www.linkedin.com/feed/update/urn:li:activity:7132731523148984320/
On November 17, Julie Rachline was invited to take part in Bsmart’s Smart Bourse program:
https://www.linkedin.com/feed/update/urn:li:activity:7131204392019472385/
Braintale was invited on November 22, 2023 on the mainstage of AI For Health Summit to share the brain biomarker perspectives during the 6th edition:
https://www.linkedin.com/feed/update/urn:li:activity:7133076715626483712/
The Trinational Health Tech Days were held on November 23 and 24, and Braintale CEO Julie Rachline took part in a round table discussion:
https://www.linkedin.com/feed/update/urn:li:activity:7133742970012356608/
The France Biotech ALS Task Force invited BrainTale on November 29, 2023 represented by Arthur BEZIE, neurology product manager, to discuss the role of biomarkers in clinical routine and research during the Colloque “SLA & maladies du motoneurone : quelles pistes pour demain?”:
https://www.linkedin.com/feed/update/urn:li:activity:7135996959877693440/
Ready for 2024, with great upcoming data, demonstration – stay tuned!
Medtech: BrainTale gathers €4.5 million to accelerate the development of its solution for diagnosis, monitoring and prediction of neurological disorders
Strasbourg, June 20, 2023
BrainTale, a French medtech deciphering white matter to enable better brain care, announces it has gathered € 4.5 million from Capital Grand Est and MACSF, as well as business angels, to accelerate its development in Europe and the United States. With this fundraising, BrainTale aims to provide physicians and pharmaceutical companies with innovative solutions to improve the diagnosis, monitoring and prediction of neurological diseases and disorders, in particular Alzheimer’s and Parkinson’s disease, amyotrophic lateral sclerosis and traumatic brain injury.
Founded in 2018 and supported from the outset by LallianSe, the life sciences integrator, BrainTale has developed and commercializes an accessible, reliable and clinically validated brain measurement digital platform solution for drug developers and physicians – neurologists, neuroradiologists and intensivists. Today, the company has gathered €4.5 million from Capital Grand Est, MACSF, business angels and healthcare professionals, supplemented by a Deeptech plan from Bpifrance Grand Est. With its first customers in Europe including hospitals (Paris Region Greater Hospitals, Hôpitaux Universitaires de Strasbourg) and biotech (Minoryx), as well as partners in the United States (Massachusetts General Hospital – Harvard), BrainTale aims an accelerating its expansion on both sides of the Atlantic.
White matter, a “new frontier” to explore
Long underestimated in neuroscience, white matter, which represents 60% of the human brain, plays a key role in its proper functioning, development, and aging, whether normal or pathological. The vast majority of brain diseases – such as Alzheimer’s, Parkinson’s, amyotrophic lateral sclerosis, adrenoleukodystrophy or the consequences of coma – are linked to primary or secondary alterations in white matter. Of note, a prediction score of recovery in comatose patients derived from white matter measurements enables physicians to significantly improve the daily care of these patients. A medical frontier that had never been explored before.
To explore this new continent of the brain, BrainTale is developing a measurement solution that improves diagnosis, monitoring and prediction with a non-invasive, pain-free, efficient and clinically validated approach. Co-founded by Professors Louis Puybasset (Intensivist, Paris Region Greater Hospitals, Paris), Damien Galanaud (Neuroradiologist, Paris Region Greater Hospitals, Paris), Lionel Velly (Intensivist, Hôpital de la Timone, Marseille Hospitals, Marseille), Didier Cassereau (Research Professor, ESPCI), Vincent Perlbarg (Engineer and PhD in Medical Physics), and LallianSe, the life sciences integrator BrainTale today employs a dozen people who develop these innovative, patented and proprietary solutions, based on international prospective, multicentric clinical development by teams of physicians, notably from the Paris Region Greater Hospitals.
A biomarker platform to measure the brain
BrainTale-care is a software medical device available in SaaS (Software as a Service) mode to any physician or drug development player based on diffusion MRI (magnetic resonance imaging). The modular platform enables the identification of white matter alterations and can provide individualized prediction scores.
The platform aims to actively contribute to the development of new drugs by drug development companies: BrainTale’s proprietary biomarkers are developed to correlate with traditional clinical scores and can make a significant distinction between active and placebo arms, improving clinical trials and supporting treatment efficacy demonstration. The potential of BrainTale biomarkers has already been demonstrated in some demyelinating diseases such as adrenoleukodystrophy (ALD).
The availability of BrainTale’s solution also enables real life understanding and observation supporting market access and paving the way for the companion biomarker principle in neurosciences.
“After 15 years of academic research and 5 years of investment, the solutions developed by BrainTale are finally available to patients and my colleagues. Responding to the unmet medical need that has been the driving force behind this ambitious project since its inception is one of the major challenges of the coming years, and BrainTale’s development is perfectly in line with this dynamic”, comments Prof. Louis Puybasset, chairman of the scientific advisory board and co-founder of BrainTale.
“LallianSe’s unfailing support and the confidence shown in us by our investors now enable us to move up a gear. Our team will be able to accelerate our innovative developments to make BrainTale biomarkers a reference for the benefit of patients suffering from neurological disorders”, adds Vincent Perlbarg, president, scientific director and co-founder of BrainTale.
“This fundraising is a decisive step for BrainTale, and enables us to look forward with ambition to our next development milestones. The commitment of Capital Grand Est and MACSF by our side reinforces our strategy to establish BrainTale’s brain measurement as a reference, in particular to enable drug developers to accelerate the development of new therapies in a more agile and efficient way, bringing a new dynamic in neurosciences”, explains Julie Rachline, president of LallianSe, and CEO of BrainTale.
“We are delighted to be able to support BrainTale in this acceleration phase alongside MACSF and an experienced and talented management team. BrainTale offers a disruptive product that combines digital health with cutting-edge fundamental research, key ingredients for success”, affirms Virginie Miath, investment director at Capital Grand Est.
“We are proud to include BrainTale in our portfolio of e-health start-ups. This investment is perfectly in line with MACSF’s strategy of helping to improve the world of healthcare. BrainTale improves the practice of healthcare professionals and the quality of care by improving the prediction of neurological diseases such as Alzheimer’s and Parkinson’s diseases. This round of financing will enable BrainTale to enter a new phase in its development,” states Sébastien Couvet, head of the MACSF Group’s e-health portfolio.
BRAINTALE PRESS CONTACTS
Laure Schlagdenhauffen laure.schlagdenhauffen@gmail.com +33 6 30 11 97 50
Anna Casal casal.anna@gmail.com +33 6 50 61 55 71
About Braintale
BrainTale is an innovative medtech company deciphering white matter to enable better brain care with clinically validated prognostic solutions. With non-invasive, sensitive and reliable measurements of white matter microstructure alterations, BrainTale offers a digital biomarkers platform to support clinical decision-making. BrainTale enables the identification of patients at risk, early diagnosis and monitoring of disease progression and the effectiveness of treatments in neurology, in particular for demyelinating diseases, amyotrophic lateral sclerosis and neurodegenerative diseases. Based on more than 15 years of research and development, BrainTale’s products are developed to meet the medical needs and expectations of healthcare professionals for the benefit of patients.
Since its inception in 2018, the company has implemented a comprehensive quality management system and is now ISO 13485:2016 certified, with a suite of products available on the European market under the European Medical Device Regulation (MDR).
For more information, please visit www.braintale.eu
About LallianSe
Pioneer and initiator of the life sciences integrator concept, LallianSe transforms innovations into economic successes through the co-construction and execution of compelling investment theses. Immersed in care, LallianSe contributes to better health through its hospital-based coworking spaces, dedicated events, Experts and Entrepreneurs in Residence, specifically mobilized for each company and its education journeys. Braintale is LallianSe’s flagship contribution.
Contac – www.lallianse.com
About Capital Grand Est
Capital Grand Est is an independent regional private equity company approved by the AMF.
Since 2012, Capital Grand Est’s 13-strong team has supported more than 60 companies in the Grand Est and Bourgogne Franche Comté regions. With close to €200 million in assets under management, spread across 5 investment vehicles, Capital Grand Est structures capital operations with different types of companies in the region, to accelerate their success. It offers young, innovative start-ups capital support at the seed stage. For more mature companies, we structure development capital operations to accelerate their growth, or transfer capital operations to support their capital evolution.
Visit: www.capitalgrandest.eu
Follow us on LinkedIn: https://www.linkedin.com/company/capitalgrandest
About MACSF Groupe
As the leading insurer of healthcare professionals, MACSF (Mutuelle d’assurance du corps de santé français) has been serving all healthcare professionals in France for over a century. It employs 1,600 people and generates sales of around 2 billion euros. True to its vocation as a professional mutual insurance company, MACSF insures the private and professional risks of over a million members and customers.
To find out more: macsf.fr
Press contacts:
Séverine Sollier – 06 14 84 52 34 – severine.sollier@macsf.fr
Annie Cohen – 06 71 01 63 06 – annie.cohen@macsf.fr
BrainTale has been invited at the AD/PD conference to present the interest of its digital biomarkers platform to improve the management of Alzheimer's disease
Strasbourg, April 6th 2023
BrainTale, a medtech deciphering white matter to enable better brain care, spin-off of the Paris Region Greater Hospitals, was invited to present with an oral communication at the AD/PD conference (International Conference on Alzheimer’s and Parkinson’s Disease and related neurological disorders) – held in Gothenburg (Sweden) from March 28 to April 1, 2023 the interest of its biomarkers platform in Alzheimer’s disease patients. Vincent Perlbarg, co-founder, scientific director and president of BrainTale, presented data obtained in the early diagnosis of Alzheimer’s disease supporting the interest of brainTale-care digital biomarker platform in patient management as well as in the development of new therapeutic approaches.
Long underestimated in neuroscience, white matter, which represents 80% of the human brain, plays a key role in its proper functioning, development, and aging, whether normal or pathological. Thus, BrainTale has been developing non-invasive, accessible, effective and clinically validated measurement and prediction tools for patients suffering from brain diseases since its creation in 2018.
Affecting more than 10 million people worldwide every year, Alzheimer’s disease incidence tends to increase. The disease is often diagnosed too late to effectively slow its progression, although white matter lesions can be detected at an early stage, notably on the myelin sheath, axons and oligodendrocyte cells.
The oral presentation entitled “Evaluation of digital biomarkers from diffusion MRI to monitor Alzheimer’s disease in the daily clinic” highlighted the study conducted on 113 subjects (66 healthy subjects, 34 patients with mild cognitive impairment, 13 patients with dementia) from 5 different institutions. This multicentre analysis showed a stratification of patients based on white matter assessments that were statistically different (p<0.05) for the three categories of subjects: decreased anisotropy fraction (AF) and increased mean diffusivity (MD).
These preliminary data highlight the sensitivity of the white matter biomarkers developed by BrainTale to specific Alzheimer’s disease, notably in early forms of the disease. It paves the way for the relevance of such biomarkers as complementary to biological markers not only for early diagnosis but also, and possibly more importantly, for disease progression monitoring. With the new version of the brainTale-care biomarker platform available since a few weeks, centers and partners equipped with BrainTale technology can now improve the management of these patients.
“The invitation for a presentation during AD/PD conference is a strong signal about the interest of our solutions to improve the management of patients in real life and to accompany the development of new therapies against these neurodegenerative diseases” comments Vincent Perlbarg “Our work allows a non-invasive, reproducible, sensitive and reliable approach of white matter alterations to better diagnose and treat Alzheimer’s disease patients in the daily clinic; the whole team is very committed to improve the management of patients” he adds.
About Braintale
BrainTale is an innovative medtech company deciphering white matter to enable better brain care with clinically validated prognostic solutions. With non-invasive, sensitive and reliable measurements of white matter microstructure alterations, BrainTale offers a digital biomarkers platform to support clinical decision-making. BrainTale enables the identification of patients at risk, early diagnosis and monitoring of disease progression and the effectiveness of treatments in neurology, in particular for demyelinating diseases, amyotrophic lateral sclerosis and neurodegenerative diseases. Based on more than 15 years of research and development, BrainTale’s products are developed to meet the medical needs and expectations of healthcare professionals for the benefit of patients.
Since its inception in 2018, the company has implemented a comprehensive quality management system and is now ISO 13485:2016 certified, with a suite of products available on the European market under the European Medical Device Regulation (MDR).
For more information, please visit www.braintale.eu
BrainTale unveils the new version of its digital biomarkers platform brainTale-care CE-marked under the MDR 2017/745
Strasbourg, March 22nd 2023
BrainTale, a medtech deciphering white matter to enable better brain care, spin-off of the Paris Region Greater Hospitals, announces the new release of its CE-marked version of the brainTale-care digital biomarker platform under European regulation MDR 2017/745. Now available to all users, this new release offers significant improvements with new features including new capabilities for patient monitoring, enhanced data and platform security, as well as optimized usage with automation and integration with integrated data flows.
Long underestimated in neuroscience, white matter, which represents 80% of the human brain, plays a key role in its proper functioning, development, and aging, whether normal or pathological. As a result, since its inception in 2018, BrainTale has been developing non-invasive, accessible, effective, and clinically validated measurement and prediction solutions for physicians treating patients with brain diseases.
Two modules are available on brainTale-care platform: brainQuant measuring quantitative, objective and reproducible white matter lesions non-invasively, and brainScore-coma dedicated to the prediction of coma recovery.
Three major features improvements are being available to customers:
- Enhanced security of the software medical device: higher security of data, data flows and optimized cybersecurity.
- Access to additional biomarkers targeting axons and myelin integrity for improved care in neurology, now available in clinical reports.
- Improved usability: automation of data flows with the medical imaging management system (PACS)
Jean-Baptiste Martini, Chief Technical Director, comments “This new version of brainTale-care is an important improvement for all our users, with superior security and usability alongside additional available biomarkers. It is a significant enrichment of the platform’s features, to allow its deployment to a growing number of customers, for the benefit of patients and industrial players“.
Beyond the renewal of the ISO13485 certification obtained early 2023, this significant update of the brainTale-care platform supports the growth and development of Braintale’s solutions of white matter biomarkers for drug development and real-life environments.
About Braintale
BrainTale is an innovative medtech company deciphering white matter to enable better brain care with clinically validated prognostic solutions. With non-invasive, sensitive and reliable measurements of white matter microstructure alterations, BrainTale offers a digital biomarkers platform to support clinical decision-making. BrainTale enables the identification of patients at risk, early diagnosis and monitoring of disease progression and the effectiveness of treatments in neurology, in particular for demyelinating diseases, amyotrophic lateral sclerosis and neurodegenerative diseases. Based on more than 15 years of research and development, BrainTale’s products are developed to meet the medical needs and expectations of healthcare professionals for the benefit of patients.
Since its inception in 2018, the company has implemented a comprehensive quality management system and is now ISO 13485:2016 certified, with a suite of products available on the European market under the European Medical Device Regulation (MDR).
For more information, please visit www.braintale.eu
BrainTale has presented the interest if its digital biomarkers platform in differential diagnosis during the 15th edition of Clinical Trials on Alzheimer’s Disease conference
BrainTale, a medtech deciphering white matter to enable better brain care, spin off from the Paris Region Greater Hospitals, has presented the “Evaluation of a clinically validated digital platform to provide Diffusion MRI biomarkers in Alzheimer’s disease” during the 15th edition of the Clinical Trials on Alzheimer’s Disease conference (CTAD 2022) held in San Francisco and online from November 29 to December 2, 2022.
The Clinical Trials on Alzheimer’s Disease conference is a meeting focused entirely on Alzheimer’s Disease Therapeutic Trials with key leaders in Alzheimer Disease research from Industry and Academia getting together and forming partnerships with the objective of speeding the development of effective treatments to fight the disease.
Long underestimated in neuroscience, white matter, which represents 80% of the human brain, plays a key role in its proper functioning, development, and aging, whether normal or pathological. As a result, since its inception in 2018, BrainTale has been developing non-invasive, accessible, effective, and clinically validated measurement and prediction solutions for physicians treating patients with brain diseases.
Alzheimer’s disease affects about 10 million people per year worldwide and its incidence rate tends to increase year after year. The disease is often diagnosed too late to effectively slow its progression, although lesions are visible at an early stage, notably on the myelin sheath, axons, and oligodendrocyte cells.
Data of 113 subjects (66 healthy patients, 34 patients with mild cognitive impairment, 13 patients with dementia) from 5 centers were processed on the BrainTale platform. The multicentric study showed that the analysis resulting from the platform processing has demonstrated statistically different white matter assessments for the 3 subjects’ categories. Results show that BrainTale biomarkers are relevant biomarkers of disease severity, and that the platform has the potential for a differentiated diagnosis perspective.
BrainTale digital biomarkers platform offers the potential to diagnose Alzheimer’s patients at an early stage and monitor disease evolution, as well as drug efficacy as already demonstrated in other neurodegenerative conditions. This potential of impact for Alzheimer’s patients, community and stakeholders has already been awarded the Robert D. Zimmerman Scientific Award 2022 by the Eastern NeuroRadiology Society.
Vincent Perlbarg, cofounder, CSO and president comments: “This preliminary work shows the importance of integrating white matter biomarkers for neurodegenerative diseases. Our data suggest a very exciting complementary approach to existing initiatives to better diagnose, and ultimately better treat Alzheimer’s disease patients”. “Our approach enables non-invasive, sensitive and reliable assessments of white matter alterations that are both available in the clinic and implemented in clinical trials to improve success rates and support efficient clinical developments.” he adds.
About BrainTale
BrainTale is an innovative medtech company deciphering white matter to enable better brain care with clinically validated prognostic solutions. With non-invasive, sensitive and reliable measurements of white matter microstructure alterations, Braintale offers a digital biomarkers platform to support clinical decision-making. Braintale enables the identification of patients at risk, early diagnosis and monitoring of disease progression and the effectiveness of treatments in neurology, in particular for demyelinating diseases, amyotrophic lateral sclerosis and neurodegenerative diseas es. Based on more than 15 years of research and development, Braintale’s products are developed to meet the medical needs and expectations of healthcare professionals for the benefit of patients.
Since its inception in 2018, the company has implemented a comprehensive quality management system and is now ISO 13485:2016 certified, with a suite of products available on the European market under the European Medical Device Regulation (MDR).
For more information, please visit www.braintale.eu
Contacts
Braintale – Julie Rachline, CEO julie.rachline@braintale.eu +33 6 62 42 03 58






